12 May 2020
Professor Mike Lewis
This is a short paper to give you an update on how science and research are progressing during the Covid-19 pandemic. Last week we looked at therapies/treatments for Covid-19 and now we should look at work on vaccines.
At the time of this note, there are 123 vaccines in various stage of development and to give the best chance of finding a ‘cure’ we will need more than 1 successful vaccine candidate. Multiple projects will give countries the option to choose or adapt the right product to their local population and also help keep costs reasonable while allowing for manufacturing challenges.
Unfortunately, the vaccine needs to be at least 60-70% effective to have an impact, and there is no guarantee any of the 123 will work. There is also a race against time where statisticians say herd immunity will effectively be achieved by the end of the 3rd or 4th wave, and a vaccine may not then be needed. More on waves and risks in the next briefing note.
There are 5 groups of vaccine we can easily group and then some odd candidates that fit no simple description. Most organisations are focussing on 3 of the groups as can be seen below.
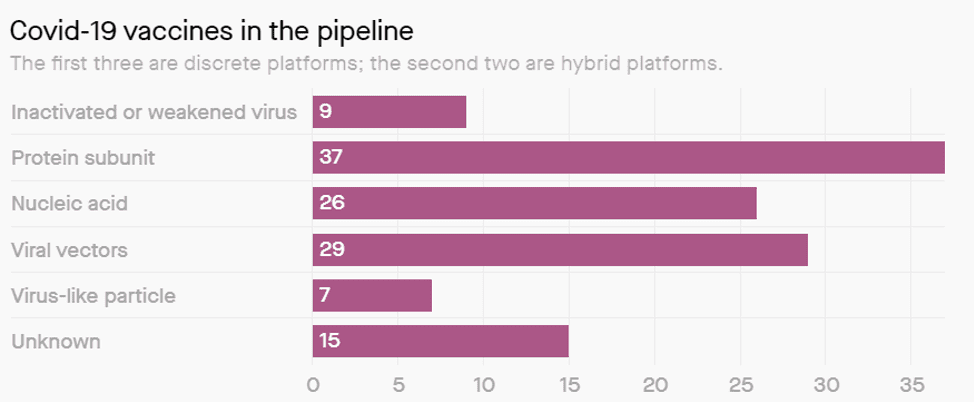
Inactivated or Weakened virus (9 candidates and 2 in trial)
Scientists have developed inactivated or weakened vaccines for illnesses like measles, chicken pox, and polio. These vaccines are tried and true but finding a new one requires a delicate balance. It has to be as close to the actual virus as possible, but not capable of replicating like it normally would.
If for some reason, the virus does start replicating, a perfectly healthy person would become sick. This is why safety testing is so critical for these vaccines. Currently, only two vaccine candidates in this category are in early clinical trials: one being developed by the Wuhan Institute of Biological Products, and one by Sinovac Biotech, which is also based in China.
Protein Sub-unit (37 candidates and 0 in trial)
Instead of showing B-cells the entire pathogen, protein subunit vaccines only show the body parts of the virus. For Covid-19, most developers are going after the spike protein that SARS-CoV-2 uses to enter our cells. The hope is that by showing B-cells that characteristic protein, they’ll be able to recognize it on the pathogen itself, too when it tries to attack you,
Protein subunits aren’t able to turn into a full-blown infection. But the immune responses they produce get weaker over time, which means that a person may require boosters throughout their life.
Nucleic Acid (26 candidates and 2 in trial)
Nucleic acid vaccines use either double-stranded DNA or messenger RNA (mRNA). These forms of genetic material contain the recipe for the desired viral proteins and cells within the body translate this foreign genetic material into target proteins, which B-cells then create antibodies against.
The advantage of this approach is that it’s relatively fast; once scientists have genetically sequenced a novel pathogen, they can isolate target proteins for the body to recreate. The challenge, though, is getting the body to actually respond to them and to produce and fold the proteins correctly.
A nucleic acid vaccine has never been approved for use. But one of the leading vaccine candidates for Covid-19 uses this approach. It’s an mRNA vaccine created by the Cambridge, MA -based company Moderna, and the US government has already invested millions in it, even though it’s still in early clinical testing. Inovio are also in Phase 1 studies with INO-4800
Viral Vectors (29 candidates and 1 in trial)
If B-cells don’t to respond to subunit vaccines or nucleic acid vaccines then an alternative is to try a hybrid approach: using other weakened or inoculated viruses to transport genetic material that codes for bits of SARS-CoV-2. The carrier virus can make its way into our cells like other infectious diseases would—but once it gets there, it produces SARS-CoV-2 proteins that generate the correct antibody response.
The only reason these vaccines would not work would be if the recipient already has some form of immunity against the knocked-out vector—making it impossible for the virus to enter our cells. One virus that scientists like to use is an adenovirus, for example, which often causes the common cold.
There are two promising vaccine approaches for Covid-19 using this platform, one by researchers at Oxford University (the ChAdOx1 product, already in trials), and one from the drug company Johnson and Johnson which goes to trails Sep 2020.
Virus like particles (7 candidates and 0 in trial)
Another that developers are exploring is a variation of subunit vaccines. Instead of getting B-cells to recognize selected viral proteins, virus-like particle vaccines introduce all the proteins on the outer shell of SARS-CoV-2. It’s like showing the outer skin only of a bubble with nothing inside it.
Currently, there are no virus-like particle vaccines in human trials—but Medicago Inc., a company based in Canada, is hoping to start theirs in July 2020
The WHO stated that there are 6 vaccines in trials but they counted Medicago which does not start human trials till July, so the real number is 5.
Chart: Courtesy of Milken Institute